Novel device to help patients after open heart surgery
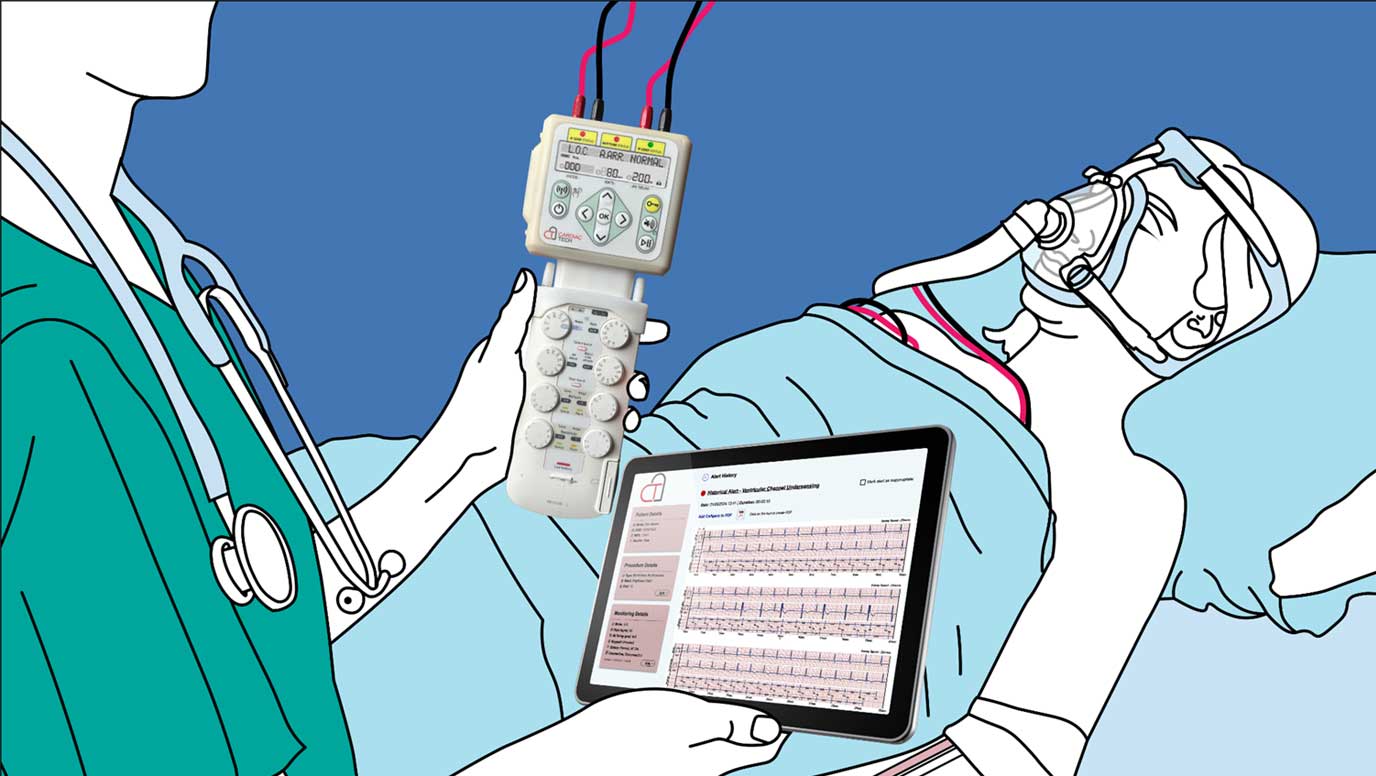
It says the device has the potential to significantly improve patient outcomes following open-heart surgery by minimising adverse events (AEs) related to temporary pacemakers.
Pace-Protect is a safety device designed to monitor all aspects of temporary pacing and report acute changes immediately to clinicians via a cloud-based platform and app. The communications electronics used in the physical system, as well as the cloud-based platform and app, have been developed by Camgenium.
Camgenium has integrated its proprietary Soft Silicon™ medical device grade two-way device communications technology for data transmission into the Pace-Protect prototype.
Soft Silicon™ is a highly sophisticated mesh architecture for communications using BLE and Wi-Fi, cellular, NFC and LORA. It establishes world class data security for patient confidentiality and high resilience to ensure individual Pace-Protects never loses contact with the cloud, ensuring safety even when a patient is moved around the hospital.
Soft Silicon™ also allows real-time patient electrogram (EGM) data to be transmitted to the clinical team at high data rates in emergencies. Pace-Protect will be the first device using a Soft Silicon™ mesh network to be deployed in a clinical environment.
“Following open heart surgery, patients often experience rhythm disturbances which can lead to serious complications,” said Will Simpson, CEO at Cardiac Tech.
“We wanted to develop a pioneering solution which enables medical professionals working in high pressure environments to be alerted of any issues immediately, bringing help to the patient before an adverse event occurs. Partnering with Camgenium is helping to bring our vision to life.”
Temporary pacemakers are currently programmed manually according to the patient’s requirements but these parameters frequently change as the heart’s conduction system recovers. These changes often go unnoticed, leading the patient’s own heart to ‘conflict’ with the impulses from the external pacemaker.
Research indicates that there are roughly 1,500 serious adverse events (SAE’s) reported each year following open-heart surgery attributable to the management of temporary pacemakers in the US alone. The Pace-Protect system addresses this unmet need for safer temporary pacemakers by providing real-time monitoring and alerts that can ultimately prevent SAEs.
“We worked incredibly closely with the team at Cardiac Tech to fully understand the needs of the patient and the clinician in the hospital,” said Dr Philip Gaffney, CEO at Camgenium.
“This enabled us to develop a user interface and user workflow that was optimised for clinical use in the hospital and met the regulated and other NHS standards for user interfaces within the highly demanding class II medical device environment.”

