Cambridge central to ClariMed’s global expansion strategy

As a human-centered medical device development and regulatory services business, ClariMed is dedicated to accelerating time to market, improving safety, and enabling innovation for pharma and MedTech teams. This approach enhances outcomes and enriches the quality of life for end users.
The company achieves this by embedding human factors and implementing user-focused, risk-mitigated services. Through these practices, ClariMed partners with clients to develop innovative medical technologies and make them accessible to the communities who need them.
The Cambridge expansion vision, championed by Louisa Harvey, Principal and Harvey Medical founder, has been realised as the operation has grown into the company’s largest lab facility with its surgical suite now operational. The rapid growth has effectively created a UK headquarters for the company at Copley Hill Business Park in Babraham, with plans to add a home simulation centre in phase 2 of the development.
“Our expansion across the UK directly responds to what we’re hearing from clients – that the traditional siloed approach to medical device development is no longer sustainable,” explains Harvey.
“As regulations evolve and devices become more sophisticated, our clients need an integrated partner who can seamlessly connect human factors insights with other components of MedTech product lifecycle.”
The new Cambridge facility was officially launched last month with a showcase event attended by senior ClariMed executives from across the US facilities and senior influencers from East of England companies – significantly including leading technology consultancies.
Amit Agrawal, Consultant Oncoplastic Breast Surgeon – who is currently working with partners to develop a device to aid surgeons – delivered a keynote address and Cambridge Cluster company AnthroTek showcased its ER-simulated abdominal cavity technology.
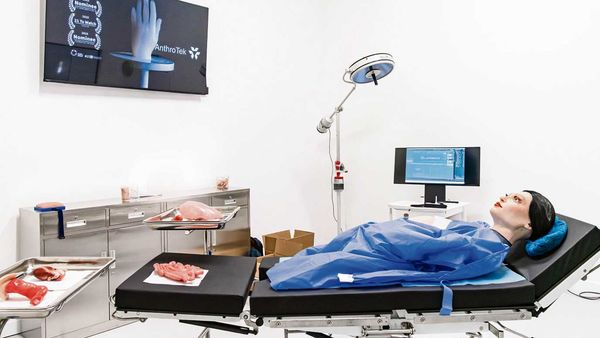
ClariMed CEO and Chair, Kelley Kendle said: “With this purpose-built Cambridge facility, we’re able to create authentic use scenarios across diverse healthcare settings, generating insights that will enhance the development of the most innovative devices and ensure they are designed to meet the needs of both healthcare professionals and patients.
“We are excited to bring this to the Cambridge community to continue to enhance the position Cambridge has in bringing the latest in science and technology to life.”
Kendle originally envisioned the ClariMed concept after 20 years running a business that provided regulatory services to biotech and pharma companies. During this time, she identified a lack of attention and service to the MedTech industry. The regulatory environment was changing for the sector with the emergence of the Medical Device Regulation (MDR), Software as a Medical Device (SaMD), and more complex systems, creating a significant opportunity for the emerging enterprise.
Simultaneously, she observed that the paradigm of care was evolving, with an expanding range of users interacting with medical technologies and increasingly diverse contexts in which these technologies were being used. These changes caught the attention of regulators and would become the foundation of the ClariMed story.
Backed by NaviMed Capital, Kendle founded ClariMed on August 12, 2022, with the acquisition of UserWise LLC in San Jose. This initial investment deliberately positioned human factors as the cornerstone of an integrated approach. Establishing a key location in the Bay Area, known for its innovative technology ecosystem, was a strategic choice that embedded ClariMed in the community and allowed it to build upon UserWise’s established reputation.
The vision presented to this forward-thinking MedTech community was to re-imagine how medical device development services could be delivered more effectively when human-centered design principles flowed through every aspect of development and regulatory strategy.
Kendle recognised that the human factors validation requirement was just one aspect and time point when user-centered design was typically considered. This approach often missed the opportunity to truly maximise the advantages of a comprehensive usability program.
While establishing a presence in the Bay Area provided an excellent foundation for ClariMed, the company recognised from the outset that global expansion was essential to address the need to develop products worldwide and accommodate the diverse environments in which they would be used. This global perspective was critical to many clients seeking a multi-regional approach in their development and regulatory pathway.
A pivotal point in the young company’s history came on January 30, 2023, when it acquired Harvey Medical – a market research and usability consultancy – which gave ClariMed offices in Cambridge, an established innovation hotbed within the UK. This move was crucial for expanding ClariMed’s global footprint and capabilities, while providing local expertise to clients worldwide.
The combined services of UserWise and Harvey Medical created ClariMed’s unique and compelling foundation – providing an end-to-end user-centered platform approach to design, development, and accessibility on a global scale.
From market insights through validation and market claims, ClariMed has, from the outset, been helping clients bring life-changing, safe, efficient products to market and ensure they can be used properly.

ClariMed has been supporting numerous clients throughout the East of England since its acquisition of Harvey Medical, which immediately established its presence in Cambridge.
“Many of our partnerships in this region represent longstanding relationships that predate our formal UK presence, with our client roster including multinational corporations, large pharmaceutical companies, and innovative startups,” Joe Dobkin, VP of Services at ClariMed, tells Business Weekly.
“The robust life sciences ecosystem across Cambridge, Oxford, and London has been a significant focus for us, complementing our work with global clients whose operations span the UK and continental Europe.”
Continued growth followed, and in August 2023, ClariMed expanded its UK presence by opening a new office in Leeds. This location strengthened the company’s European footprint and positioned it within another thriving healthcare innovation ecosystem, strategically located in the North of England.
This strategic approach has allowed ClariMed to swiftly establish a multinational presence across the US, UK, and EU. The company set up headquarters in Chadds Ford, PA – outside of Philadelphia – and continues to pursue both organic growth and strategic acquisitions.
Since its founding in 2022, ClariMed has scaled rapidly, now employing 65 people across five offices. The company is positioned for continued growth as it works toward its vision of becoming the worldwide leader in human-centered regulatory and development services for medical products by adding quality and digital services to its portfolio.
The new Copley Park facility in Cambridge adds 10,000 square feet – including ClariMed’s second-floor gallery viewing rooms – to the company’s global footprint of five locations with simulation labs. The Copley Park facility is the global company’s second-largest facility – second only to San Jose. And the Cambridge expansion may not be the end of the growth spurt.
Harvey notes the broader implications of this integrated approach: “This integration is particularly crucial given that most medical products are used and marketed globally, requiring deep expertise in varying regulatory requirements across the US, UK, EU and other regions.
“Our global team understands these nuanced differences and can navigate complex submission processes while maintaining consistency in human-centered design principles.
“We’re not just growing geographically, but strategically building integrated capabilities that eliminate the coordination burden manufacturers have traditionally shouldered when working with multiple specialised vendors,” she concludes.

